Existentialist philosophers like Jean-Paul Sartre and Albert Camus emphasized the inherent
vulnerability and seeming absurdity of human existence, painting a picture of beings thrust
into
a world rife with uncertainties. This vulnerability, especially to disease, serves as a
clear
reminder of our mortality. Yet, it is precisely this vulnerability that fuels our profound
sense
of self-preservation and an insatiable thirst for knowledge. Plato, for instance, believed
that
knowledge was akin to seeing the light, a way out of the cave of ignorance. This pursuit of
understanding is intrinsically linked to our survival instincts, compelling us to decipher
the world around us to better navigate its myriad challenges. Immunology, in this context,
is not a mere academic discipline that emerged in a vacuum. Instead, it stands as a product
of centuries of human curiosity, observation, and the drive for survival. I recognize the
vulnerability and challenges of preserving life, and I am driven to contribute to the
advancement of Immunology, a field intimately connected with the very basis of disease
like no other discipline in modern biology.
2019 National Scholar Recognition, Valledupar, Cesar, Colombia
Commemorative Metal Plaque Donated to the School

2022 BMS End-of-Year Global Townhall, Cambridge, MA, USA
Presentation: Increasing the Safety and Efficacy of PR3 Autologous Chimeric Antigen Receptor T-Cell Therapy for the Treatment of ANCA Associated Vasculitis
2023 Biogen Science is Brewing Event, Cambridge, MA, USA
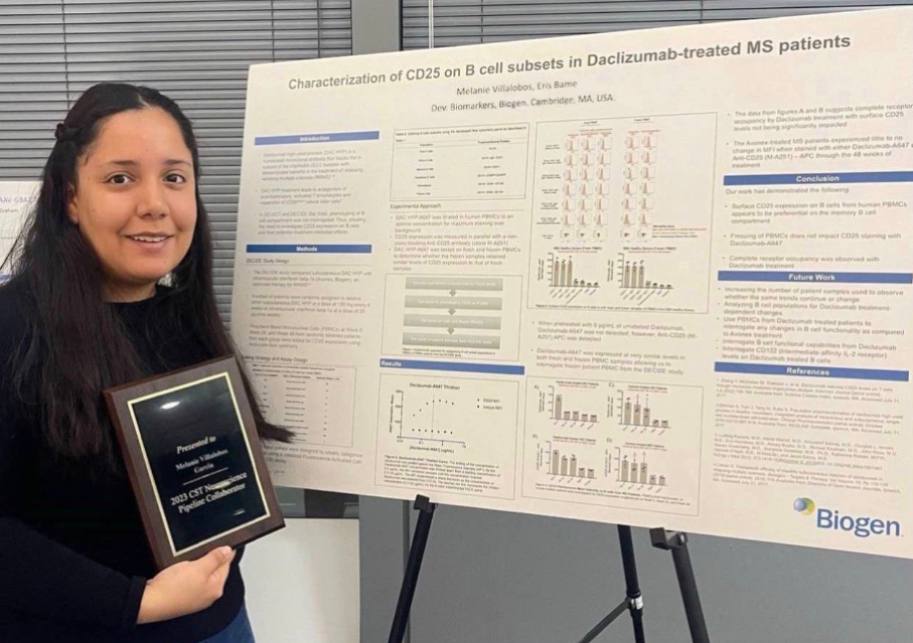
Despite reducing the frequency of MS relapses, Daclizumab was associated with serious and sometimes fatal side effects, including inflammatory brain disorders and severe immune-mediated conditions. The mechanisms behind these adverse effects are believed to be linked to its immunomodulatory action. By blocking CD25 on T cells, Daclizumab may have disrupted regulatory T cell functions and caused an uncontrolled immune response. It may have also had unforeseen effects on other immune cells, particularly B cells, the other key player in MS pathobiology, contributing to inflammation and autoimmunity. However, the exact mechanisms leading to these adverse effects are still not fully understood. This study aimed to investigate the impact of Daclizumab on the B cell compartment, specifically on CD25 expression. We believe that understanding these effects on B cells will provide critical insights into the broader immunological impact of Daclizumab and its associated risks.
The data used in this study was derived from the DECIDE study conducted by Biogen in 2017, which compared Daclizumab with interferon beta-1a in the treatment of RRMS. This randomized clinical trial provided a robust dataset for analysis. We used contemporary data analysis techniques including data transformation and analysis pipelines to perform a detailed retrospective analysis on the characterization of CD25 on B cell subsets in Daclizumab-treated MS patients. The preliminary data analysis revealed significant receptor occupancy and consistent CD25 expression patterns in memory B cells. This suggests a potential impact of the drug on long-term immunity and immune memory. Since Daclizumab was associated with serious adverse effects, understanding its interaction with memory B cells could also shed light on possible reasons for these effects. Multiple sclerosis is a complex disease with an immune component. Studying how an immunomodulatory drug like Daclizumab affects disease helps in understanding the underlying immune processes in RRMS. This can lead to better strategies for managing or treating MS, beyond the scope of Daclizumab. Multiple sclerosis is a complex disease with an immune component. Studying how an immunomodulatory drug like Daclizumab affects disease helps in understanding the underlying immune processes in RRMS. This can lead to better strategies for managing or treating MS, beyond the scope of Daclizumab
IMMUNOLOGY2023, Washington D.C., USA
Dean’s Academic Excellence Fellow (travel grant and fellowship)









